Tailoring thermal radiation
through light recycling
Light emitted from glowing hot objects (such as the Sun and incandescent light-bulbs) is of tremendous utility, yet a large fraction of the radiated energy doesn’t make it to end use. The rest is lost to unwanted wavelengths: for example, in solar cells, the mismatch between the Sun’s spectrum and the cell’s absorption profile limits efficiency; in incandescent light bulbs, most of the energy is wasted as heat. Because of this, there is potential in finding a way to shift emission from unwanted to useful wavelengths.
The prevalent way of doing this is through patterning the emitter with periodic nano structures such as photonic crystals. However, a major challenge with this approach is the difficulty of keeping such delicate structures stable at very high temperatures.
In our work, instead of embedding such structures into the emitting surface, we surround the emitter by special nanophotonic “filters”, designed to transmit desirable light and reflect unwanted light coming from all directions. In this way, the combination of a thermal emitter and such a filtering structure (top image) enables a very high degree of light recycling.
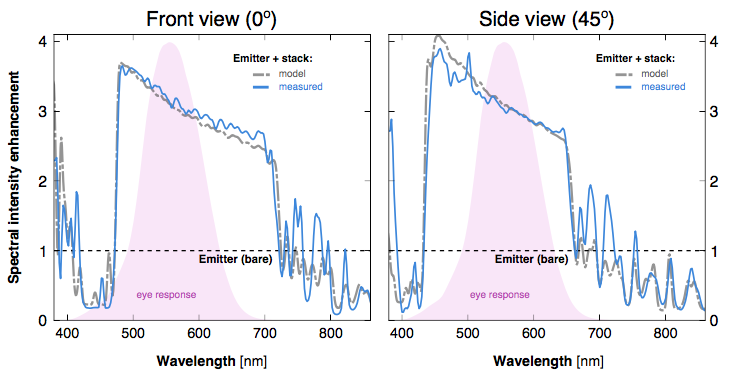
Experimental demonstration of thermal emission tailoring. More visible light is emitted when nanophotonic filters surround the metallic emitter.
To demonstrate this concept, we focused on a traditional incandescent filament. It is an inefficient light source because most of the electrical energy is wasted as infrared light, invisible to human eyes.
Our goal was to recycle these infrared emissions, while allowing the visible light to pass. In our experiment, we show that—for the same amount of input power—much more light is emitted in the visible spectrum when nanophotonic filters surround the tungsten emitter. Such devices are made of abundant materials and are particularly amenable to large-scale fabrication, making it a potentially inexpensive, scalable technology.
For more information, see our paper below:
- “Tailoring high-temperature radiation and the resurrection of the incandescent source”, O. Ilic, P. Bermel, G. Chen, J.D. Joannopoulos, I. Celanovic and M. Soljačić Nature Nanotechnology, 11, 320-324 (2016).
Additional materials are available here.
The following news articles nicely explain this work:
- “Recycling light”, written by Paola Rebusco (MIT).
- “A nanophotonic comeback for incandescent bulbs?”, by David L. Chandler from MIT News Office (front page Spotlight).
In addition, this work was featured in:
The Economist, Science/AAAS, Science Friday (radio), BBC News, NBC News, Physics Today, Optics & Photonics News, Smithsonian Magazine, Popular Mechanics, National Geographic, Engadget, Gizmodo, Nanowerk, CNET, Phys.org, Photonics Spectra, The Independent, The Telegraph, IEEE Spectrum, Science Daily, Tech Times, Photonics.com, Focus.it, EurekAlert, and elsewhere.
Is this work meant to displace LED or flourescent lights?
No, LED bulbs are an amazing, fastly-improving technology that people should be using. In fact, they are already approaching very high efficiencies. Our work isn’t about competing with current lighting technologies, but about understanding the fundamental physics of tailoring thermal emission from a hot object (more on that below). We picked the example of (very challenging) incandescent emission as a show-case of the power of nanophotonic techniques for tailoring thermal emission.
Under what circumstances and how soon could this approach achieve very high efficiencies?
The results we describe in our paper refer to optimal conditions of a nanophotonic structure with a large number of layers of several different materials and no light leakage. In terms of optical design and photonics theory, we understand the process to make that happen. Nevertheless, there are important challenges and considerations that include fabricating such a structure and ensuring mechanical stability of the device. For any commercial developments, the issues of mass scale production and reliability would have to be addressed.
Are there any commercial partners with your project?
We are not contemplating commercialization at this point.
What is the background and implications of this research?
At the core of this work is the idea of tailoring thermal radiation. At lower temperatures, we know how to do that – we directly nano-pattern the surface of the emitting object to get the output light that we want. Now, at higher temperatures, this approach doesn’t work, because these nano-structures start to deteriorate.
Because of this, we turn to the concept of “light recycling”, i.e. to pass through the desirable wavelengths and reflect/recycle the unwanted ones.
This is a general concept that applies to any high temperature thermal emitter, and the choice of desirable vs. undesirable wavelengths will change based on application. In order to test this concept, we decided to try it with one of the hottest thermal emitters available – an incandescent filament. There, the experiment showed that light recycling is indeed possible and that it works the way we expected.
The implications of this are also relevant for energy-conversion applications such as thermo-photovoltaics. In a thermo-photovoltaic device, external heat causes the material to glow, emitting light that is converted into an electric current by an absorbing photovoltaic element. Tailoring that light, to better match the PV element, could improve conversion efficiency.
Wasn’t this done before?
Improving the efficiency of an incandescent light bulb by surrounding it with an IR filter goes back to the 1980s (we cite some of the work in our paper, and similar work was done in industry, including Philips, GE: US Patents 4,535,269, 4,547,704, 4,588,923, 4,949,005). In our case, detailed modern numerical optimizations that give us exceptional optical properties, combined with a planar, re-designed, emitter, allow for a substantial increase in efficiency. Our goal was to demonstrate light recycling in a planar geometry, as it would be relevant for more advanced optical structures (such as 2D or 3D photonic crystal thermal emitters and filters) and other applications.
What is the purpose of this research?
It’s about understanding the science of how (and to what extent) thermal emission from high temperature sources can be tailored through this concept of light recycling, which might have many different potential applications for energy conversion (including concepts such as thermo-photovoltaics).
Additional materials are available here.
How do you compare the efficiency of your device to CFLs/LEDs?
For an incandescent source, its luminous efficacy can be expressed as
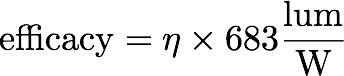
where η is the “luminous efficiency” that we calculate in our paper (Eq. 2). To get an estimate for how LEDs and CFLs compare when this definition of efficiency is used, we scaled their lum/W values by that factor (683). That gives the numbers reported in the paper.
One can, more appropriately, instead compare efficacies. To do that, we do the inverse and simply multiply the “luminous efficiency” for an incandescent source by 683, to get its lum/W value.
With this in mind, a typical incandescent bulb would have efficacy of ~15 lum/W, a store CFL bulb may usually be anywhere in the 40-100 lum/W range, and a store LED bulb anywhere in the 40-140 lum/W range (note that state-of-art LED chips can go over 200 lum/W, and are constantly improving). For our experimental, proof-of-concept, device efficacy is estimated at about 45 lum/W (6.6%). Under theoretically optimal conditions (discussed above), they could, in principle, reach much higher.
Yet another metric for a lighting source may be the fraction (percentage) of input power that is converted into visible light. For this quantity, the generally accepted numbers are: incandescent (~5%), CFL (~15-20%) and linear fluorescents (~20-25%). For our proof-of-concept experimental device the estimated number is about 16% (and could, in principle, go over 80% under theoretically optimal conditions).
As we previously mentioned, however, the purpose of our device is to demonstrate a high degree of recycling of thermally emitted light, especially in a planar geometry - not to be a competitor to LEDs/CFLs.